
Eudamed is a database that will be used to monitor both the safety and performance of medical devices distributed in Europe and it has been introduced in parallel with the EU MDR 2017/745. It will be a great tool to improve quality, transparency and regulatory operations for medical devices manufacturer.
The European Commission decided that its database of medical device information (Eudamed) will be onlineonce when all modules are fully functional, so the commission has decided to launch the database simultaneously for both medical devices and in-vitro diagnostics in May 2022, in parallel with the new EU MDR 2017/745.
A Commission spokesperson said the delay will now coincide with the implementation date of the in-vitro diagnostic regulation to make sure all systems are in sync.
Eudamed database will be used for a lots of different functions, in particular:
- Registration of devices
- UDI-database
- Registration of economic operators
- Notified bodies accreditation and certificates
- Clinical investigations
- Vigilance and post-market surveillance
- Market surveillance
In principle Eudamed database has different goals. The most important ones are the following:
- Enable the public to be informed about the medical devices currently on the market, including their manufactures and certificates.
- To ensure that UDI-related information is available
- Ensure the public is informed about clinical investigation
From the regulation point of view, the functions of the EUDAMED are mentioned in Article 33 of EU MDR 2017/745, where it is reported:
Eudamed shall include the following electronic systems:
(a) the electronic system for registration of devices referred to in Article 29(4);(b) the UDI-database referred to in Article 28; (c) the electronic system on registration of economic operators referred to in Article 30; (d) the electronic system on notified bodies and on certificates referred to in Article 57; (e) the electronic system on clinical investigations referred to in Article 73; (f) the electronic system on vigilance and post-market surveillance referred to in Article 92; (g) the electronic system on market surveillance referred to in Article 100.
Regarding the registration of UDI-related information in the EUDAMED database, recently the European Commission established UDI and Device Datasets for Medical Device and In-Vitro Diagnostic devices: all the information can be found at this link. This is an example of the UDI-related information to be submitted in EUDAMED for Medical Devices:
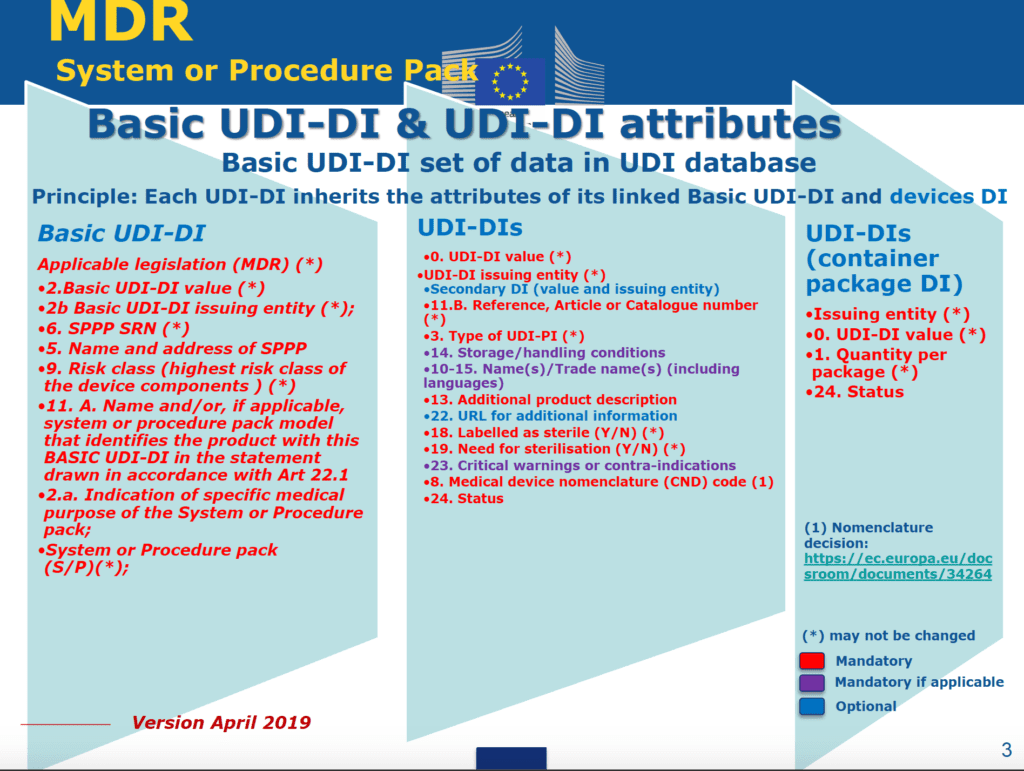
The registration of UDI information in EudaMed database allows EU to be more alignbed with FDA regulation, where all the UDI information shall be registered in the so-called GUDID database.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
