FDA Digital Health Devices Regulation – Pre-Cert Program
FDA has launched the so-called Software Pre-Cert Program. The goal is to provide a specific regulatory pathway for market access which is more suitable for digital health devices, in particular SaMD (Software As Medical Device). Firstly, FDA has already in place specific guidelines to help the manufacturer for regulatory submission for software-related medical devices, for example this specific guideline for SW in medical device.
First of all, the goal of this program for digital health devices is to develop a regulatory model that will be used in the future as a regulatory path for SaMD, including software based on artificial intelligence and machine learning. This is to help manufacturers which have demonstrated a strong culture of quality and organizational excellence.
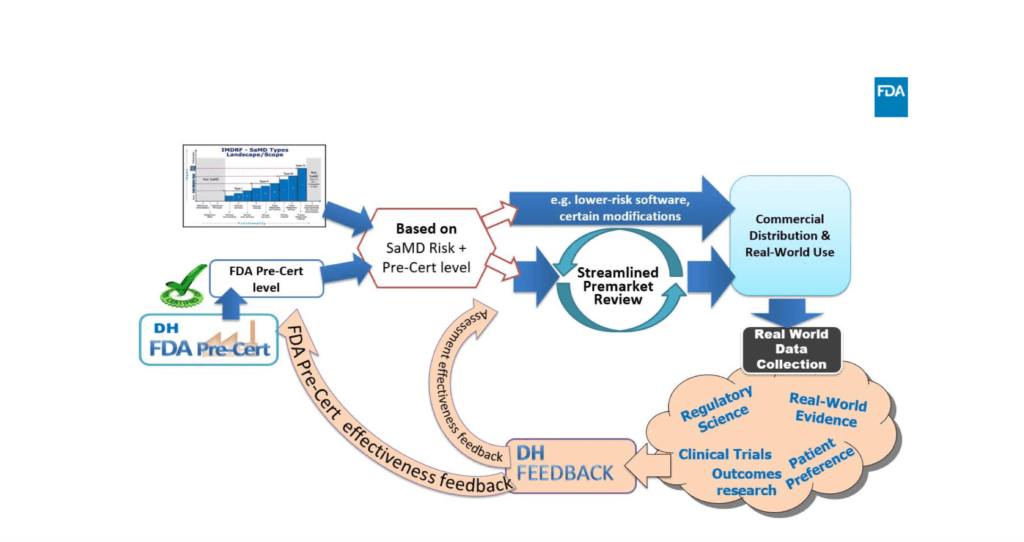
Moreover, FDA Digital Health Devices Regulation – Pre-Cert Program will help the agency to provide a more adequate regulatory model for digital health devices. Above all, the goal is to be more aligned with the current trends in the medical device sector, especially for SaMD and the related use of novel technologies like artificial intelligence and machine learning.
For instance, the program is based on 5 excellence principles:
- Product Quality,
- Patient Safety,
- Clinical Responsibility,
- Cybersecurity Responsibility,
- Proactive Culture
The Pillar of the Pre-Cert Program
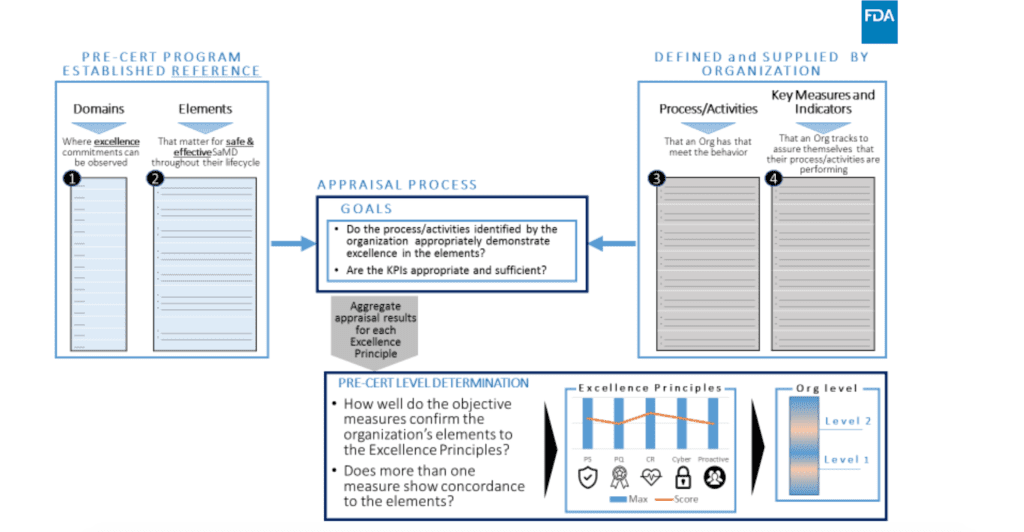
Moreover, the evaluation of how the company demonstrates excellence in these 5 principles is performed by evaluating specific principles which have been identified as guidance for excellence in the elements mentioned before:
- Leadership, and Organizational Support
- Transparency
- People
- Infrastructure and Work Environment
- Risk Management: A Patient Safety Focused Process
- Configuration Management and Change Control
- Measurement, Analysis, and Improvement of Processes and Products
- Managing Outsourced Processes, Activities, and Products
- Requirements Management
- Design and Development
- Verification and Validation
- Deployment and Maintenance
- Real World Health Analytics
- Product Performance Analytics
Currently, the participants to the programs are the following:
- Apple
- Fitbit
- Johnson & Johnson
- Pear Therapeutics
- Phosphorus
- Roche
- Samsung
- Tidepool
- Verily
In conclusion, FDA Digital Health Devices Regulation – Pre-Cert Program, along with regulation for wellness devices, established a new of way of thinking for digital health devices. The regulation of software-related medical device is a general trend through different regulators around the world.
The program represents a very good framework based on which future medical device regulations could be based.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
