GXP requirements became more and more important in the last years. In this post, we provide an easy guideline to GXP compliance.
In the last years, the complexity of the regulations further increased, along with the complexity of medical products (Pharma & Medical Devices). Specifically, this is mainly due to the introduction of new technologies which required some changes from the regulators.
What GXP stands for?
GxP is a series of quality guidelines and regulations created to ensure that bio/pharmaceutical products are safe and adhere to quality processes during the whole life cycle of the products.
G: Stands for good
x: Variable
P: Stands for practices
Here below, a summary of the main GxP:

The Fundamentals of GXP Compliance
First of all, three basic concepts stand at the base of any GXP regulations:
- Traceability: the ability to reconstruct the development history of a drug or medical device.
- Accountability: the capacity to determine who has contributed what to the development and when. In other words, it is the capacity to identify the contribution of each individual during the development process.
- Data Integrity: the reliability of data generated by the system.
Summary of GXP Regulations
GXP : Good Manufacturing Practice (GMP)
Good Manufacturing Practice refers to the regulations related to the authorisation and controls on the manufacturing of drugs, medical device, active pharmaceutical ingredients (API).
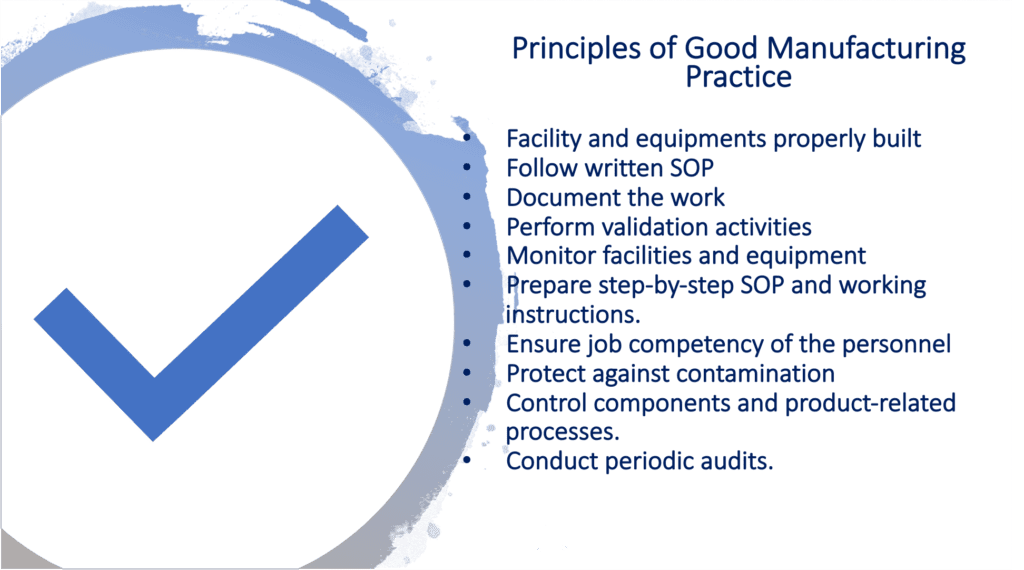
In United States, GMP are enforced by FDA under the so-called Title 21. For medical device manufacturers, there are as well specific requirements for the quality system (QSR : Quality System Regulation). This is very similar to ISO 13485 requirements.
In addition, for the pharmaceutical sector in Europe, there are three regulations that define the GMP requirements:
- Regulation No. 1252/2014 and Directive 2003/94/EC, applying to active substances and medicines for human use;
- Directive 91/412/EEC applying to medicines for veterinary use
The EU GMP guidelines provide interpretation of these principles and guidelines.
GXP : Good Laboratory Practice
GLP is a managerial quality control system covering the organisational process and the conditions under which non-clinical health and environmental studies are planned, performed, monitored, recorded, reported and retained (or archived).
GLP : Historical Overview
New Zeland and Denmark were the first countries, in the early 1970s, to introduce GLP regulation, as response to malpractice in research and development (R&D) activities by pharmaceutical companies. In addition, the Organization for Economic Co-operation and Development (OECD) defined the GLP principles. Since then, OECD has helped to spread GLP principles in many different countries.
Currently, for United States GLP are defined within 21CFR part 58, whereas for European the regulation is handled by the OECD. (here the whole set of regulations).
GLP Applicability
Commonly, Good Laboratory Practice are associated to the Pharmaceutical Sector, however these regulations apply to all the non-clinical safety studies related to head the environment. In other words, this means that GLP apply to pharmaceutical products, additives for human and animal food, cosmetics and veterinary medicinal products.
GLP Principles

GXP : Good Clinical Practice
Firstly, good clinical practice (GCP) is an international ethical and scientific quality standard for designing, recording and reporting trials that involve the participation of human subjects. Moreover, the GCP principles form clinical trials on medical devices are as well described on the ISO 14155.
GCP : Historical Overview
For Europe, two directives describes the requirement for the conduction of clinical trials:
- the ‘Clinical Trial Directive’ (Directive 2001/20/EC);
- the ‘GCP Directive’ (Directive 2005/28/EC).
For United States, FDA website contains a list of all applicable regulations related to the conduction of clinical trials.
Harmonization have been performed by the ICH (International Council for Harmonisation) to ensure to have an harmonized guideline for clinical trials. Currently the most important countries of the world, including US and European Union, recognises the GCP guideline from ICH.
GCP Principles
Above all, GCP are based on 13 principles which are summarised with the picture below:


GXP : Good Documentation Practice
Firstly, good documentation practice plays an essential role in whole life science business and it is a fundamental practice to reach high level of regulatory compliance. GMP requirements already integrate good documentation practice. However, there is a specific WHO guideline that describes the requirement for data and record management.

For instance, different ISO standards and other regulations, such as ISO 13485 and FDA QSR , contain requirements for good management of documentation. If the documentation is handled through a specific software, careful validation shall be performed.
Conclusions
In conclusions, GXP refers to a series of guidelines applicable to the life science sector covering different topics ranging from manufacturing, clinical studies, laboratory tests, documentation management and more. For instance, this article reports an overview of the main regulation related to GxP, along with the most important principles which stand at the base of these regulations.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
