The quality manual is one of the most important documents for a company with a quality system in place, and in particular if the company is ISO 13485 certified.
ISO 13485:2016 contains specific requirements for the quality manual, that are outlined below. Specifically, the quality manual shall contain, at least:
- The scope of the Quality Management System with details and justification of any exclusion of clauses of ISO 13485 standard;
- Reference to the procedures of the Quality System;
- Description of the interaction of the different processes of the quality system;
- The structure of the documentation of the QMS
According to FDA regulation 21 CFR 820, there is no specific requirements to have a Quality Manual. However, the presence of a Quality Manual within the organization surely helps auditor to gain an overview of the Quality System in place.
Let’s now have a look in details to all the contents and section that should be present in a Quality Manual.
Scope of the Quality Management System: A key point for Quality Manual
Defining the scope of the Quality Management System is a key step in for the development of any management system. The scope shall reflect the activities performed by the organization, e.g. design, manufacturing, sale and or distribution of specific medical devices. It is important to do not omit any specific activities that would impact on the devices on the market as scope of the Quality System.
Reference to the procedures of the Quality System
To be compliant with ISO 13485 the quality manual shall have at least reference to all the procedures involved in the Quality System.
This part is relatively easy, and it involves the inclusion of the list of all the procedures within the quality system of the organization. Sometimes if is useful to create a link between the processes of the organization and the procedures. See section below for more details.
QMS Processes Interactions
Another important factor to have a quality manual compliant with ISO standard is the documentation of the interactions of the different QMS processes.
The interactions between the processes of an organization is usually documented with a process map. It defines the different processes and their related interconnection. So for example, usually on the top of the process map there are management responsibilities / management review as it is the highest form of control for the processes of the Quality System.
Then, we will have all the processes and related relations. For example design process is connected with purchase as it provides the specification for the specific item to be bought; purchase, in tern, is definitely linked to manufacturing operations, which in turn is linked with sale process.
Here below, an example of process ma that takes in consideration only the design and manufacturing process:
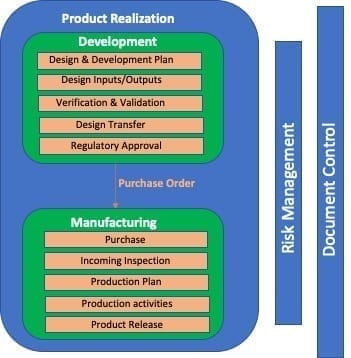
As said before, it makes sense to have a correlation between QMS process and procedure, so to know to which process a specific procedure is applicable to. A table to document this link is surely enough.
Structure of the Documentation in the Quality Manual
Moreover, the structure of QMS documentation must be documented in the Quality Manual. This is a standard requirements and typically it is covered by inserting the classic pyramid that outlines the structure of the documentation.
Below, an example of the classic structure of quality documentation.
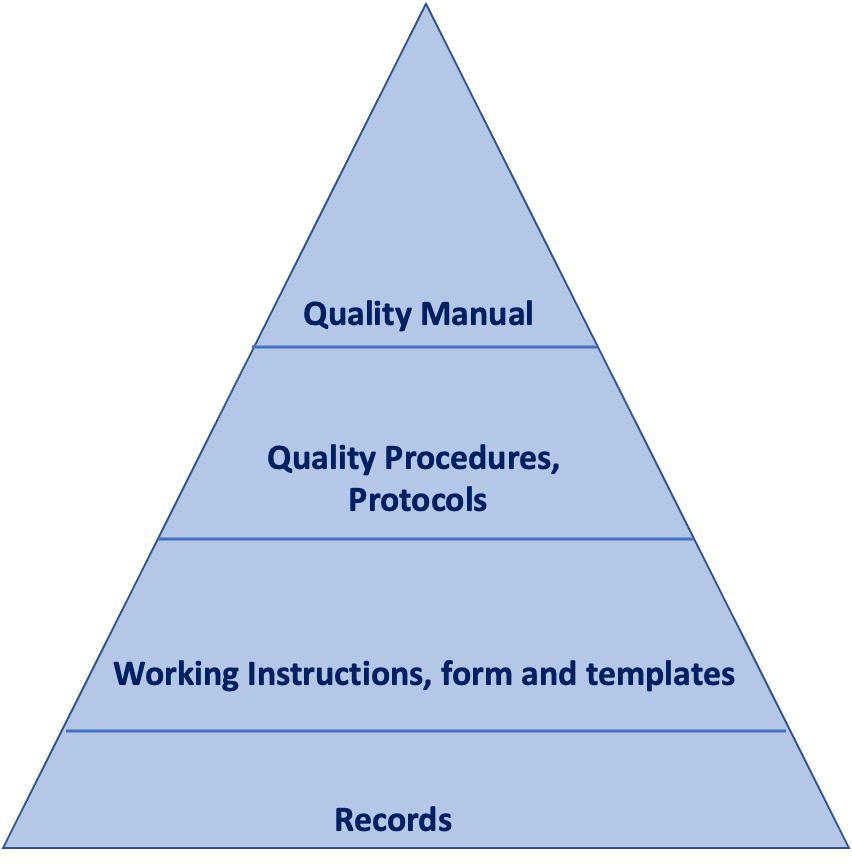
-
 Document Control Procedure€64,00
Document Control Procedure€64,00
Conclusions
In conclusions, these are the most important requirements to be addressed in the Quality Manual for an organization dealing with a Quality System, especially within the medical device sector. As mentioned above, FDA does not have specific requirements for Quality Manual , however it remains an essential document from which any external stakeholder could have an overview of the Quality System of the organization.
Internal Audit
If you need to check the compliance of the Quality Manual to the applicable regulatory requirements, the best way is to use the Internal Audit Process. You can download the Internal Audit Procedure for 4EasyReg DocShop to ensure to have a fully compliant Internal Audit process in place for your organization.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!

