The UDI (Unique Device Identification) is a system used to uniquely identify medical device sold in a specific country, for example in United States. FDA started the implementation of UDI back in 2014 for Class III devices and completed in September 2020 for Class I device.
The UDI is a fundamental component of medical devices and very important for the design process of a Quality System, as well for QMS requirements related to traceability and labelling.
4EasyReg published an e-book containing a comprehensive overview of the labelling requirements for EU and FDA, which takes in consideration new or updated ISO standards related to labelling and IFU.
Advantages of the UDI System
The implementation of a Unique Device Identification system for medical device brings a lot of advantages for all the stakeholders of the medical device sector: manufacturers, regulatory authorities, health professionals and patients. Among the advantages, there is surely a more accurate traceability and the possibility to batter trace adverse events reports and facilitate actions on the field such as recall.
The use of a fully standardised identification system is another great advantage, with benefits as well for the logistic operation linked to distribution activities.
How FDA UDI system works
The UDI coding system for US market is provided by accredited agencies fro FDA which have been selected to provide the specific codes used to build the UDI system. For the US market, there are three FDA-accredited agencies that can provide UDI codes:
- GS1
- Health Industry Business Communications Council (HIBCC)
- ICCBBA
These agencies are issuing UDI codes according to FDA rules and based on a specific UDI format which has been defined in this specific document.
The Structure of the UDI
The UDI system follows a specific structure which, as mentioned above, changes based on the issuing agency and based on the information that needs to be encoded by the automatic reader.
The UDI system is composed of the following parts, as described in the scheme below:
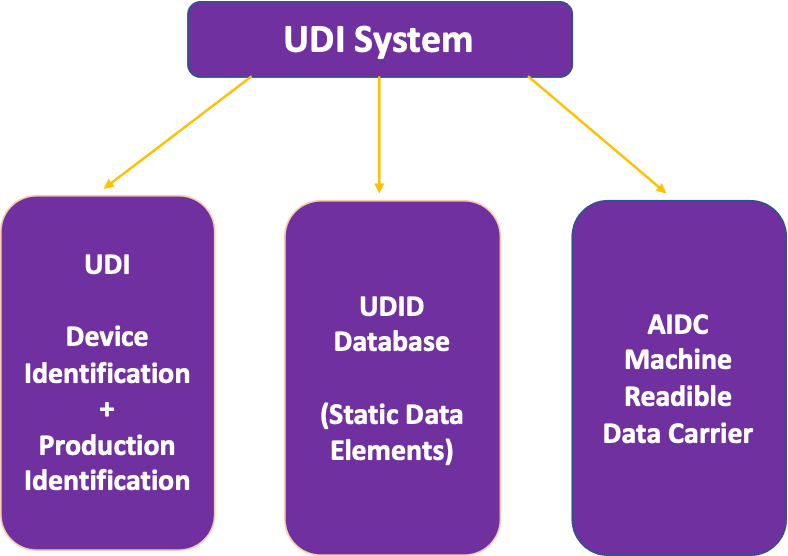
First there is the UDI coding system, which is formed by device related static data (Device Identifier, DI) plus dynamic data which are called Production Identifier (PI). Then there is UDID Database, which is the place where the static data elements of the UDI system are recorded for each medical device. For FDA, this database is GUDID and it is publicly available. Lastly, we have the data carrier, a machine readable system that encodes for the DI and PI information.
UDI Coding System
As mentioned before, the UDI codes are formed by a Device Identifier and a Production Identifier. The DI portion is based on the GTIN (Global Trade Item Number) and is identifying the specific device model. There are different length for the GTIN, based on the number of digits. However, independently from the length, all the GTIN system has the following structure:
- a company prefix, which is the part of the code that is recognising the organization
- a sequential number, which will be different on each item
- a key controller number, which is calculated based on the previous sequence number.
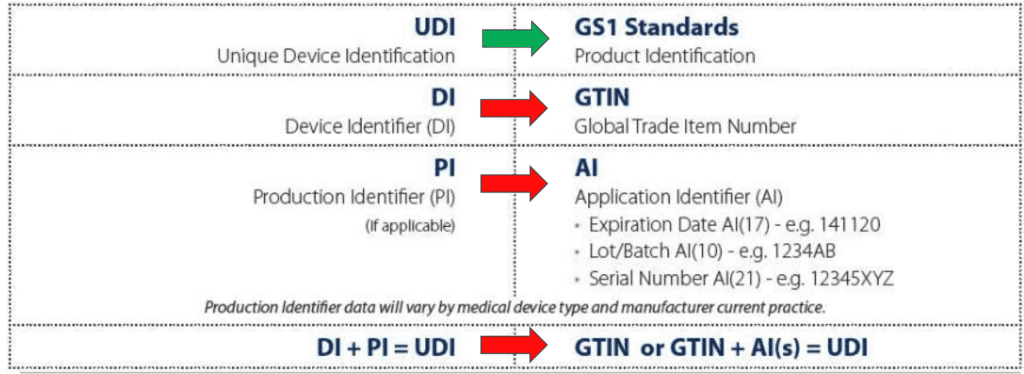
The PI portion is the variable part of the UDI and it can include different parts, each of them in a specific format, as mentioned in the scheme above:
- Expiration Date
- Lot/Batch Number
- Serial Number
The UDID Database
Each Unique Device Identification system (US, EU) has a specific database where the static information of UDI along with information on manufacturer and other information needs to be recorded. For FDA, this database is called GUDID whereas for Europe there will be the Eudamed.
There is a lot of documentation for GUDID Database which contains a list of information such as (for example):
- Product Code
- FDA Listing Number
- GMDN Code
- The Production Identifier
- If the device contains latex
- If it prescription or Over the Counter medical device
- etc etc
The Data Carrier
As mentioned before, the UDI coding system shall be accompanied by a machine reader data carrier that shall encode for the information reported in the label.
There are different data carriers, which can be used, as per picture reported below:
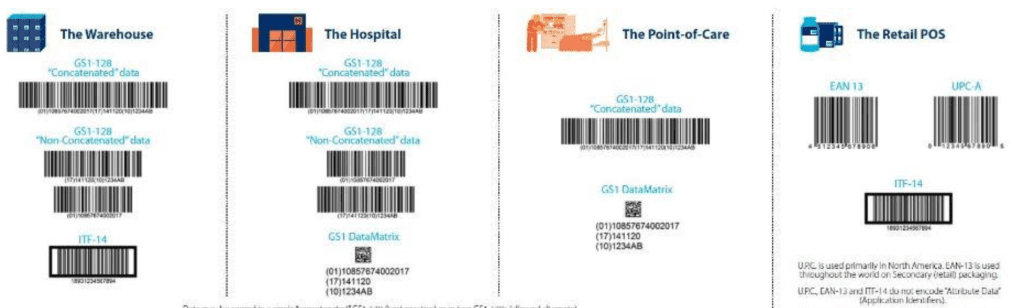
The specific choice of data carriers depends on multiple factors, including the class of risk of medical device, the information to be encoded and if there is any constraint related to supply chain or logistic and of course from the accredited agency of choice.
If we take in consideration always GS1 as example, here is a scheme of how the data carrier could change based on multiple factors
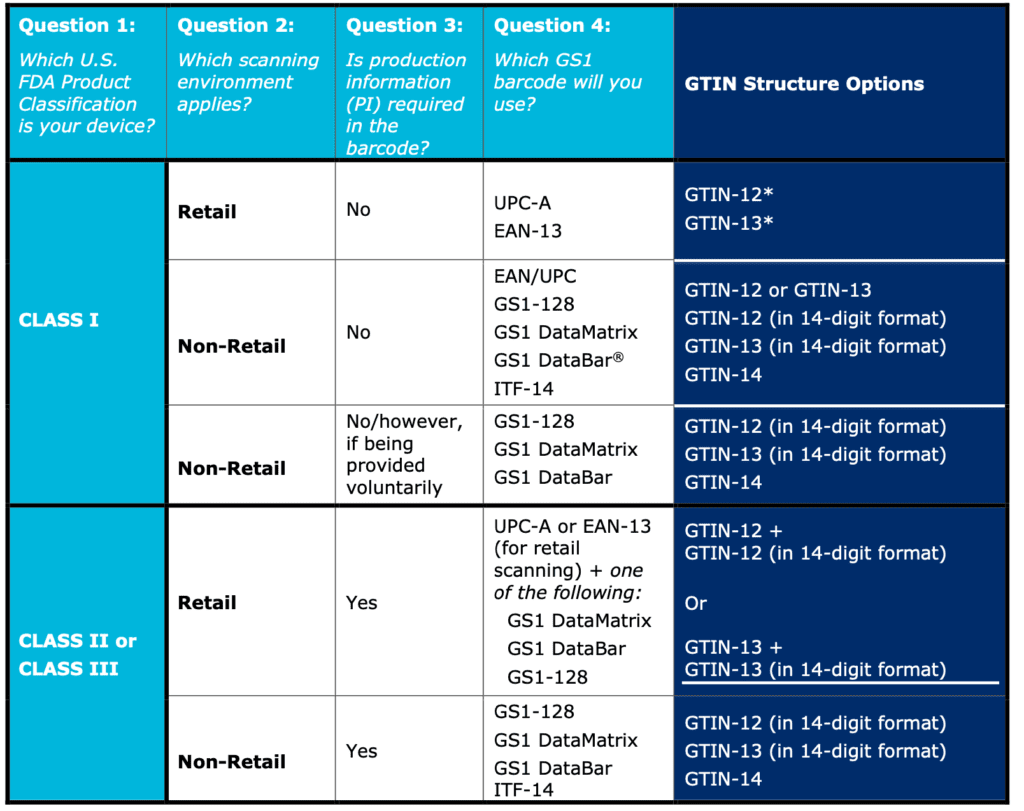
UDI Management Procedure
4EasyReg has published a UDI management procedure including the UDI-related requirements associated to FDA QSR and EU MDR 2017/745.
The contents of this UDI Management Procedure are the following:
- UDI Format
- UDI Carrier
- UDI Issuing Agencies
- General Process for UDI creation
- Submission of UDI information on the UDID database (GUDID or Eudamed)
- UDI Labelling Requirements
- UDI Requirements according to EU MDR 2017/745
Here at 4EasyReg DocShop you can find a procedure for UDI management, covering requirements for United States and European market, thus including FDA and EU MDR 2017/745 requirements. This is a 9 pages document, fully editable in word, which can be adapted to your organisation and your quality management system.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
