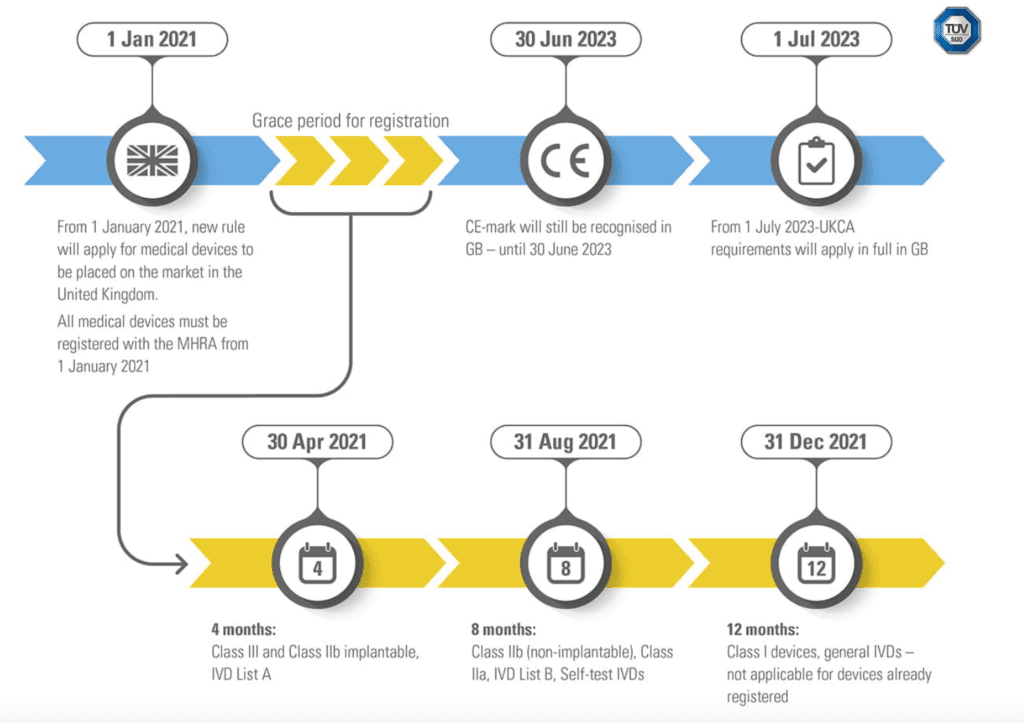
Due to Brexit, UK will not recognised CE mark for medical devices and an UKCA mark and the related UKCA conformity assessment will be mandatory to place medical devices in United Kingdom. Brexit is having big impact on product certification process and the related design process for medical device manufacturers. The UKCA (UK Conformity Assessed marking) will replace the EU requirements for CE marking and it currently constitutes the product marking requirements to distribute products in UK. The CE mark will continue to be recognised; therefore, it is possible to continue to place your products on the market in the UK until 30 June 2023.
The UKCA mark introduces as well other requirements that manufacturers shall respect in order to place medical device in UK market. All the medical devices need to register with the MHRA (UK’s Medicines and Healthcare products Regulatory Agency) starting from 01 January 2021; a grace period of registration has been decided and the length of this grace period depends from the class of risk of the device (high class of risk devices have a shorter grace period).
It is important to mention that the UKCA is not going be recognised within European Union.
Moreover, for all the manufacturers located outside United Kingdom, a UK responsible person is required; the address of the UK Responsible Person shall be within UK.
The UKCA mark will be issued by UK Approved Body and the conformity assessment and modalities for obtaining the UKCA mark will mainly depend from
UKCA Mark and Conformity Assessment
The conformity assessment for UKCA mark depends from the type of devices and the related class of risk. There are three different regulations which are at the base of the UKCA mark and related certification process:
- general medical devices: Part II of the UK MDR 2002
- active implantable medical devices: Part III of the UK MDR 2002
- in vitro diagnostic medical devices (IVDs): Part IV of the UK MDR 2002
As mentioned before, different Classes of devices have different ways to obtain the UKCA certification.
Class I devices
A declaration of conformity with the requirements mentioned in the UK MDR 2002 shall be prepared by the manufacturer. Furthermore, in case the device is sterile or it has measuring functions, review of the technical documentation by an UK-based Approved Body shall be performed.
Class IIa devices
Declaration of conformity with the requirements mentioned in the UK MDR 2002 shall be prepared by the manufacturer. Moreover, application to an Approved Body is needed and conformity assessment shall be performed.
Class IIb and III devices
For class IIb devices, the manufacturer shall:
- a Part II of the UK MDR 2002, Annex II (as modified by Part II of Schedule 2A to the UK MDR 2002) audit of full quality assurance system or;
- a Part II of the UK MDR 2002, Annex III (as modified by Part II of Schedule 2A to the UK MDR 2002) type-examination plus either option 1, 2 or 3 given for the Class IIa devices above
For Class III devices, the conformity assessment is very similar.
What Product Marking will get you Where?
Medical devices with CE Marking
If a medical device has the CE Mark, it can be sold and distributed in Europe and in Northen Ireland. It can be used as well within Great Britain, but only until 30 Jun 2023, after which GB will not recognised anymore the CE marking and the UKCA certification process will be mandatory.
Medical Devices with UKCA mark
Medical devices with UKCA mark can be used in Great Britain starting from 01 January 2021 and it will be mandatory from 01 July 2023. However, the UKCA mark is not recognised in Northen Ireland and in European Union.
Medical Devices with UKCA and CE marking
In this situation, the medical devices can be sold and distributed within European Union, as well as in Great Britain and in Northen Ireland.
UKCA mark and Labelling Requirements
The labelling requirements related to Brexit and UKCA will depend on the situation of the medical devices. For medical devices placed in Great Britain based on CE certification/CE marking, no additional UKCA mark shall be applied to labels. Manufacturers outside UK shall appoint an UK Representative Person (UKRP) but it is not mandatory to identify the UKRP the labelling.
Instead, in case of medical devices bearing the UKCA mark, obviously the labelling shall include the UKCA mark; moreover, in this case, for manufacturers outside UK the UKRP shall be appointed and identified on either labels or IFU.
