The benefit risk analysis is one of the key elements of the risk management process for medical devices and, more in general, a key element for any quality system. We have already been talking extensively of risk management activities, including ISO 14971:2019 and the related technical report ISO/TR 24971:2020. We have also discussed about risk management requirements for other life science sectors, for example the pharmaceutical sector and the related ICH Q9 guideline.
-
 Risk Management Procedure€64,00
Risk Management Procedure€64,00
With the introduction of the EU MDR 2017/745, additional specific requirements related to risk management and more specifically to risk benefit analysis are required. As we all know, the link between risk management, PMS and clinical evaluation is essential for a quality system compliant with the EU MDR.
In this post we will go through the requirements associated to the risk benefit analysis in the framework of the European Medical Device Regulation 2017/745.
Let’s start with some definitions. According to Article II of the EU MDR, “benefit-risk determination” means the analysis of all assessments of benefit and risk of possible relevance for the use of the device for the intended purpose, when used in accordance with the intended purpose given by the manufacturer.
It is important to understand the concept of clinical benefit, as it is strictly related to the benefit risk analysis. Clinical benefit is defined as the positive impact of a device on the health of an individual, expressed in terms of a meaningful, measurable, patient-relevant clinical outcome(s), including outcome(s) related to diagnosis, or a positive impact on patient management or public health.
According to the EU MDR, the benefit-risk determination shall be part of the technical documentation. Infact, Annex II clearly states that the technical documentation shall include :
- The risk benefit determination, in other words the necessity to accept all the risks associated to the device when they are outweighed against the evaluate benefit
- Evidence for the establishment, implementation and documentation of risk management activities; among these activities, there is as well the determination of the benefit/risk ratio.
Moreover, as mentioned before, the link between PMS (post-market surveillance) and risk management became more and more important. In fact, in Annex III related to Technical Documentation on Post-Market Surveillance, it is clearly mentioned that the post-market surveillance plan shall include “suitable indicators and threshold values that shall be used in the continuous reassessment of the benefit-risk analysis and of the risk management”
Moreover, also the post-market clinical follow-up plan shall include the modalities by which the continued acceptability of the benefit risk ratio is evaluated; this is reported in the Annex XIV part B of the EU MDR 2017/745.
-
 PSUR report Template€64,00
PSUR report Template€64,00 -
 Postmarket Surveillance Plan Template€64,00
Postmarket Surveillance Plan Template€64,00
How to Perform a Risk Benefit Analysis
The methodologies that can be used for the risk-benefit determination are reported in the ISO/TR 24971:2020, section 7.4.
The benefit risk analysis shall be performed for those risks which are considered not acceptable based on the criteria established in the risk management. USually the risk benefit analysis shall be performed by experienced personnel, typically a multidisciplinary team including medical, clinical or product experts.
The Benefit Estimation
The ISO 14971 provides extensive guidelines for the determination and analysis of risks associated with the medical devices; however, the determination of the benefit is less straightforward.
The benefit related to a medical device is related to the extent of improvement of health expected from its use. The benefit can be of different types, including positive impact on clinical outcome, or user’s quality of life, or more in general a positive impact on public health. Sometimes benefits can be described and determined based on the benefit that a specific patient population will experience.
The benefit can be estimated from different factors such as:
- the performance expected from the device during clinical use;
- the clinical outcome expected from that performance;
- benefits resulting from the use of similar medical devices;
- factors relevant to the risks and benefits of other diagnosis or treatment options.
Often it is difficult to apply a rigorous approach in the risk-benefit determination. For this reason, it is necessary to take in consideration some specific aspects that could help to simplify the analysis. For example:
— the type of expected benefits for the patient or other people (e.g. the medical device is life-saving or essential in a given medical scenario);
— the magnitude of the expected benefits (e.g. the degree to which the patient will experience the therapeutic or diagnostic benefit);
— the probability that the patient will experience the expected benefits (i.e. the likelihood that the medical device is effective in treating or diagnosing the patient’s disease or condition); and
— the duration of the expected effects (i.e. how long the benefit is expected to last for the patient).
-
 Risk Management Package€149,00
Risk Management Package€149,00
Overall residual risks & risk benefit analysis
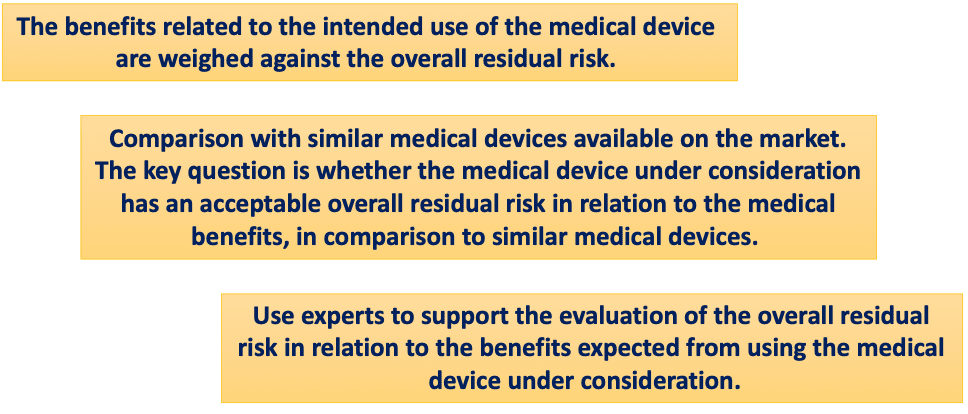
The benefit risk analysis is linked with the so-called evaluation of residual risks. The criteria used for the evaluation of the overall residual risks are different from the acceptability criteria of each single risk. In fact, the criteria used to evaluate individual risks usually include limits for the probability of occurrence of harm with a particular severity. The criteria used to evaluate the overall residual risk are often based on additional elements, such as the benefits of the intended use of the medical device.
The possible approaches that can be used for the overall residual risk evaluation are reported in the scheme below:
Conclusions
In conclusion, we have been going through some specific requirements related to the risk-benefit analysis , which an essential part of risk management activities for medical devices. In particular, we analyzed the risk-benefit analysis in the framework of the requirements associated to the EU MDR 217/745 whereas in the last part of the article we provided some practical approaches to perform the risk benefit analysis.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
