The concept of custom-made devices is surely not new and many competent authorities have specific requirements in place to regulate the manufacture and distribution of these types of medical devices.
In this articles, we will go through the requirements for custom made devices according to the European Medical Device Regulation 2017/745.
Definitions
The EU MDR specifies within the Article 2 the definition of a custom made device. Specifically:
- is specifically made in accordance with a written prescription of any person authorised by national law by virtue of that person’s professional qualifications; which gives
- specific design characteristics provided under that person’s responsibility; and
- is intended for the sole use of a particular patient exclusively to meet their individualconditions and needs.
The characteristics of a custom made devices are rather clear from the definition mentioned above.
It is important to mention that there is a different between custom made devices and adapted devices, with the latter being devices that are produced in large quantities and then are adapted or somehow shaped to fit the specific customer needs. These types of devices (for examples, wheelchairs, hearing aids, etc) are not considered custom made devices.
What are the main requirements for custom-made devices
As it s mentioned in the specific MDCG guideline focused on custom made device, the manufacturer of these types of devices shall basically be compliant with the vast majorities for EU MDR requirements, although there are few specific differences that now we will go through.
First of all, a custom made device shall surely respect all the applicable standards in terms of safety and performance, and the manufacturer is the responsible entity to ensure this compliance. In other words, Annex I of the EU MDR related to GSPR (General Safety and Performance Requirements) shall be fully respected.
Secondly, the manufacturer shall have a quality system in place that shall be compliant with article 10 of the MDR, as previously discussed in this post at QualityMedDev. Specifically, it is essential that the implemented quality system addresses all the post-market surveillance requirements, and in particular specific communication method shall be implemented in order to properly receive feedback on quality, safety and efficacy of the device on the market.
Other standard QMS processes shall always be properly in place, such as risk management, clinical evaluation and, as mentioned before, post-market surveillance.
Conformity Assessment for Custom-Made Devices
Conformity assessment for custom made devices is described in the Annex XIII of the regulation. Here in the scheme below there is a summary of the obligations of manufacturers in order to place a custom made device in the European market.
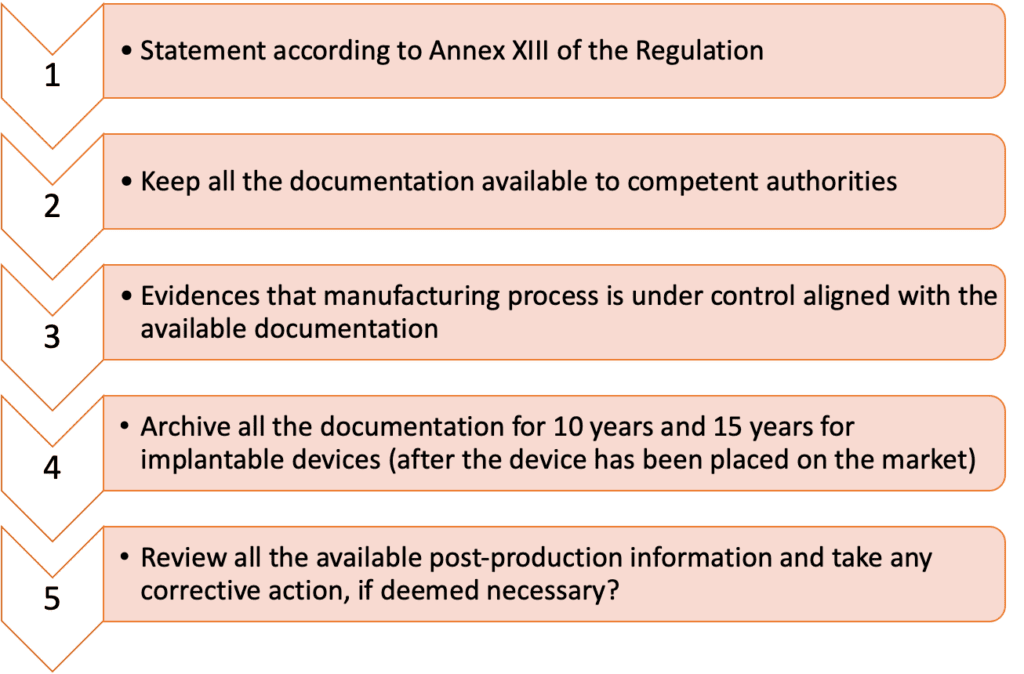
First of all, in place of the Standard Declaration of Conformity, manufacturers of custom made devices shall prepare the so-called Annex XIII statements, that shall include a series of information. This statement shall be made available to the particular patient or user identified by a name, an acronym or a numerical code.
Documentation related to the design, safety, manufacturing (including address of the manufacturing sites) shall be maintained by the manufacturer and made available upon competent authorities requests.
Moreover, evidences of the conformity the manufacturing process with all the retained documentation linked to the devices shall be maintained by the manufacturer (point 3).
Retention period plays also a fundamental role. Manufacturers of custom made devices need to retain the product-related documentation for 10 years after placing the device on the market; if the device is implantable, the retention period is 15 years.
Finally, the last requirement is related to post-market surveillance process. Specifically, the MDR states:
The manufacturer shall review and document experience gained in the post-production phase, including from PMCF as referred to in Part B of Annex XIV, and implement appropriate means to apply any necessary corrective action. In that context, it shall report in accordance with Article 87(1) to the competent authorities any serious incidents or field safety corrective actions or both as soon as it learns of them.
In other words, it is necessary to collect all the relevant post-market surveillance information and evaluate the necessity to implement any corrective action. Moreover, it is an obligation of the manufacturer to communicate any adverse events to the relevant competent authorities.
Class III Implantable Custom Made Devices
For implantable Class III custom made devices, the conformity assessment is different and it involves quality management system certification, that could be obtained using two different strategies:
- Annex IX QMS assessment
- Annex XI part A – Conformity assessment based on product conformity verification.
Further information on the conformity assessment procedures shall be found in a different article at QualityMedDev website.
Annex XIII Statements
The Annex XIII defines specifically what need to be contained in the statement necessary to place a custom made devices on the market. QualityMedDev has prepared a specific template for this statement that can be easily download from our QualityMedDev Shop.
This template is fully aligned with the requirements of Annex III and , after having included all the relevant information, it will become the essential documentation necessary to place a custom made devices on the market.
Conclusions
In conclusions, we went through all the requirements associated to custom made devices according to the European Medical Device Regulation. It is essential to meet all these requirements in order to place these types of devices in the European market.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
