One of the most important regulatory submission processes for medical device manufacturers is the so-called 510k submission, used by FDA to authorize the introduction on the US market of medical devices for which an equivalent device is already present in the US market.
The 510k process is quite complex and highly demanding in terms of design documentation for the specific medical device.
At QualityMedDev we have already been extensively discussing the topics related to design control, the structure of the medical device file and the other sections of the technical documentation that are of fundamental importance to provide evidence of the safety of the device on the market (verification & validation, biocompatibility, usability, post-market surveillance).
In this section we will go through the main concepts associated to the 510k applications, the contents of the submission and the different types of 510k it is possible to have.
Premarket Approval vs. 510k
Typically the Food and Drug Administration allows the introduction of medical device in US market by means of different routes:
- The 510(k) process
- The De Novo Process, for devices using specific new technologies for which no equivalent devices are present on the market.
- Premarket Approval (PMA) for class III. Usually the PMA requires clinical and laboratory studies and a detailed process to determine safety and effectiveness.
The 510(k) process, which is also known as Premarket Notification, requires medical device manufacturers to notify the FDA at least 90 days before marketing their new products unless the devices are exempt from 510 (k) requirements.
The 510(k) regulatory submission is based on the concept of “substantially equivalence”: the demonstration that a device is equivalent to an already marketed device. In other words, when the FDA clears a device through 510(k), it is not examining if the product is safe or effective for use in patients, because since the device is equivalent to an already existing device, then it is assumed to be safe from patient point of view.. It is just agreeing with the maker’s claim that the device is similar to another device already on the market.
Types of Premarket Notifications
There are basically three different types of Premarket Notification 510(k) that can be submitted to the FDA:
- Traditional
- Special
- Abbreviated
The Special 510(k) Program is intended to facilitate the submission, review, and clearance of a change to a manufacturer’s own legally marketed predicate device that is already authorized for commercial distribution through 510(k) clearance or through a granted De Novo classification request.
Insteal, the abbreviated 510(k) process relies on the use of guidance documents, special controls and voluntary consensus standards. In an Abbreviated 510(k) submission, manufacturers shall provide summary reports based on the use of guidance documents and/or special controls, or declarations of conformity to recognized standards, to facilitate the FDA’s review of a submission.
Contents of the 510k Submission
In this section we will present an overview of the contents of a classic 510(k) submission. The list of documentation and contents to be included in a classic 510k submission can be summarized below:
- Medical Device User Fee Cover Sheet (Form FDA 3601)
- Table of contents
- Cover sheet and cover letter
- Comparison of Intended Use & Indications for Use
- 510(k) Summary
- Declarations of Conformity and Summary Reports
- Device Description
- Substantial equivalence (comparison, labeling, IFU and Indication for USe of predicate devices
- Proposed Labeling (Labeling overview, draft label, draft IFU)
- Sterilization and shelf life, if it is deemed applicable, based on the type of devices (Sterilization and Shelf-life Overview, Sterilization Validation Report, Accelerated Age Testing)
- Biocompatibility (unless the device is a SAMD): Biocompatibility Overview and related tests based on the specific endpoints needed to be evaluated.
- Software-related documentation based on IEC 62304: SW Overview, level of concern, SW description, Device Hazard Analysis, Software Requirements Specification (SRS), SW Architecture, Traceability Analysis, Software Development Environment Description, Verification and Validation Documentation, Revision History and list of unresolved anomalies.
- Electrical Safety and electromagnetic compatibilities: electrical safety and EMC test reports
- Non-clinical tests: Benchtop Performance Testing Overview, Shipping Validation.
Obviously, not all the documents may be applicable to your device: for example electrical safety and EMC testing are not applicable for non-active medical devices; it is up to you to properly identify the applicable requirements associated to your device and include the related documentation.
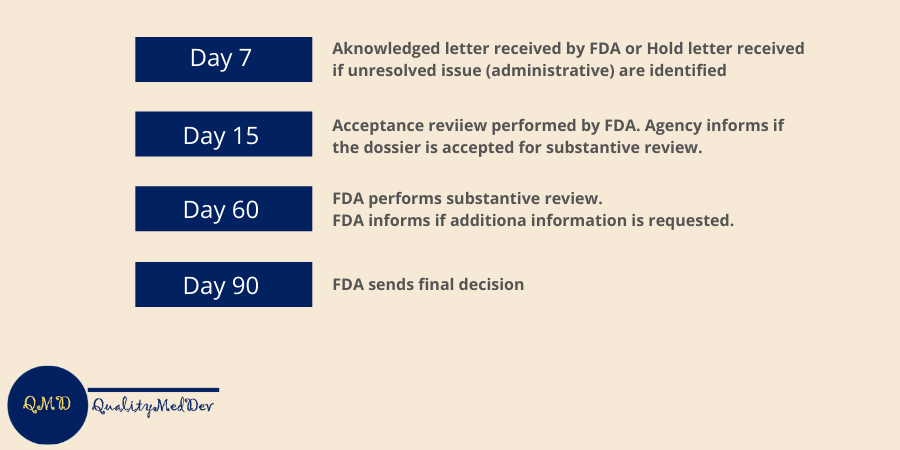
How long does the 510k process take?
The process takes at leas 90 days but it may take much longer, expecially if the documentation is not complete or if the agency is asking additional information needed to support the application. The overall timeframe can be summarized with the scheme below:
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
