In the last years, the reprocessing of medical devices started to play a critical role and additional requirements have been requested by competent authorities and regulators to ensure the safety of the reprocessed device; in this context ISO 17664 defines the requirements associated to the processing of medical devices, particularly in the context of the information to be provided by the manufacturer for reprocessed medical devices.
We have already been talking about cleaning validation with a particular attention to the pharmaceutical sector. With this post, we will focus more on the medical device sector to discuss the information to be provided by manufacturer to ensure adequate processing of devices. As we know, in the last years, the importance of labelling and information to be provided by manufacturer largely increased, especially in the framework of the labelling requirements associated to the EU MDR 2017/745 and the related standards, such as ISO 15223 and the new ISO 20417.
As we know there are medical devices that need to be processed before they can be considered ready to be used. This includes medical devices that are intended for reuse and require processing before they could be safely reused (thus they need to be cleaned, disinfected and/or sterilized).
The ISO 17664 provides guideline to medical device manufacturers for the information that need to be provided to ensure safety and effectiveness for the intended use for the devices that require cleaning followed by disinfection/sterilization between uses.
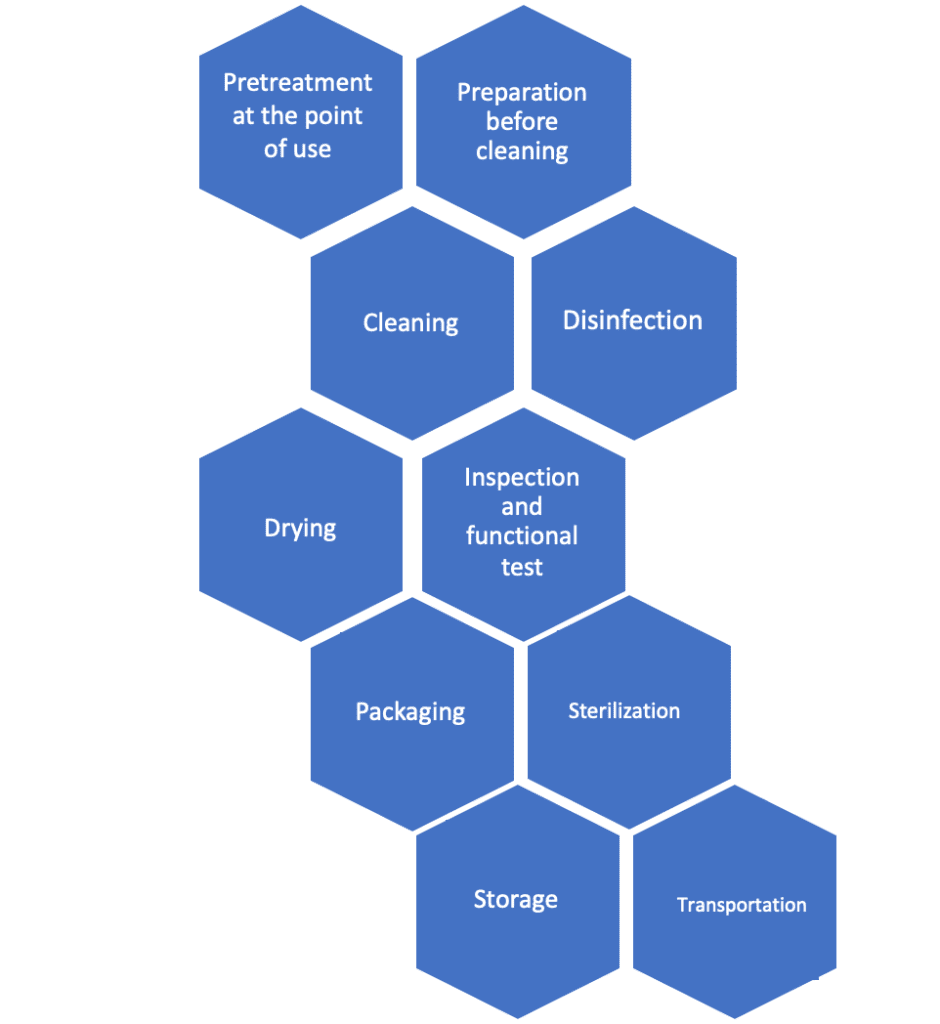
Processing activities in the framework of ISO 17664 include the following:
- Pre-treatment at point of use
- Cleaning, disinfection, drying
- Inspection and functional testing
- Packaging, sterilization, storage and transportation.
Definitions according to ISO 17664
Before going through the main requirements associated to ISO 17664, let’s go for some definitions that are fundamental to fully understand the context of this ISO standard. These definitions are directly taken from ISO 17664.
- Cleaning : removal of contaminants from an item to the extent necessary for further processing r for intended use.
- Disinfection : process to remove, destroy or deactivate microorganisms on surfaces of products to a level previously specified for its intended use.
- Processing : activity including cleaning, disinfection and sterilization (if necessary and applicable) to prepare a new or used healthcare product for its intended use.
- Processor : organization and/or individual with the responsibility for carrying out the actions necessary to prepare a new or reusable healthcare product for its intended use.
Requirements for Validation of the Process according to ISO 17664
The process which is identified in the information provided along with the medical device shall be validated. The goal of the validation is to ensure that the identified process is able to properly process the medical device to make it suitable to be used for its intended use.
In the framework of this validation activities, objective evidence shall be provided to ensure the process is properly validated; thus, a specific validation strategy shall be defined, depending on the type of processing, the specific medical devices and their intended use.
Risk Management in the framework of ISO 17664
We have been extensively discussing about risk management for medical devices, including the main standard ISO 14971 and the technical report ISO/TR 24971.
In the framework of ISO 17664, it is essential that the information to be provided are the outcome of the risk analysis process performed by the manufacturer.
Information to be provided for processing of medical devices
The ISO 17664 provides guideline on the information to be provided for all the different types of processing for medical device. In the context of this article, we will discuss only selected examples of processing methods, among the ones which have been mentioned in the previous section.
Specifically, we will discuss:
- General Requirements on the information to be provided for medical device processing
- Requirements for pre-treatment at the point of use before processing
- Requirements related to cleaning
- Requirements related to disinfection.
Sterilization and packaging have not been included in the discussion of this post since the requirements associated to these processes have been discussed within other articles at QualityMedDev.
General Requirements
There are some general requirements which are not dependent from the type of process applied to the medical device.
First of all, it is essential when disinfection or sterilization is the terminal process, the validated method shall be clearly either to reduce the risk of transmission of infections diseases or to ensure adequate level of sterility.
When the processing method is critical to the maintenance of the intended function of the device or critical to the safety of the patient, it is necessary to provide the information:
- Details of the process steps
- Necessary equipment
- Specification for process parameters and related tolerances.
Furthermore, it is very important that if the manufacturer is aware that the processing method might have an impact on the devices in terms of degradation or shelf life, specific restrictions or limitation shall be provided to the processor.
Requirements for pre-treatment at the point of use before processing
In case the medical device needs pre-treatment at the point of use before cleaning, the following information shall be provided:
- Description of the technique that needs to be used
- Specific controls that need to be performed
- The critical time that it is necessary to have between the use of the device and the pre-treatment
- Any support systems and requirements for transportation of the device, including the transportation steps.
Requirements related to Cleaning according to ISO 17664
Automated cleaning shall be used unless it is not feasible with the specific medical device; in this case, a validated manual cleaning shall be specified.
If automated cleaning is doable though the use of a washer disinfector compliant to ISO 15883, the information to be provided are limited to those parameters specific for the device (accessories, process chemicals, temperature etc). Otherwise, if this is not the case, it is necessary to provide information on the following:
- Process description and processing parameters
- Description of the required accessories
- Quality of the water to be used
- Chemicals required, including information on the related concentration.
- Methodologies to be used for rinsing.
Very similar information shall be provided in case of manual cleaning.
Requirements related to disinfection
The requirements related to the disinfection process are quite similar to the ones specified for the cleaning. Also in this case, information on at least one automatic method for disinfection shall be provided, unless the device is not compatible with automatic disinfection. In this latter case, manual disinfections can be used.
Again, the ideal solution would be for automatic disinfection to use an equipment which could fit the requirements associated to ISO 15883. If this is not feasible, the information to be provided are similar to the ones mentioned for the cleaning process, such as:
- Process description and processing parameters
- Description of the required accessories
- Quality of the water to be used
- Chemicals required, including information on the related concentration.
- Methodologies to be used for rinsing.
- Contact time between the devices and the disinfecting agent
- Any available information on any potential incompatibility with a specific disinfecting agent.
Conclusions
In conclusions, with this article we have been going through the overall structure and the main requirements of the ISO 17664, related to the information to be provided for the processing of medical devices to be performed before use.
As we might understand, medical devices processing before use is a critical and it can have a big impact on safety of patient. Cleaning and disinfections are often performed to avoid potential contamination of the patient with microbes or other pathogens; processing, moreover, could also require sterilization of the device. Given the criticality of this process, particular attention and more strict requirements have been requested to medical device manufacturer by the regulators, to further increase the safety of the patient and the quality of the devices on the field.
