ISO 15223-1:2021 new symbols for medical devices
In this paragraph, we will talk about the update of ISO 15223-1 that will introduce new symbols to add in the labelling of medical devices. Specifically, along with ISO 20471, the new ISO 15223 is a fundamental standard to support the manufacturers in preparation of labelling and accompanying documents. For instance, after the new regulation on risk management, this is an important update for medical device industry.
QualityMedDev published an e-book containing a comprehensive overview of the labelling requirements for EU and FDA, which takes in consideration new or updated ISO standards related to labelling and IFU. In this overview of labelling regulations, the following topics are covered:
- In depth overview of the labelling Requirements according to EU MDR 2017/745, with a specific table summarising all the needed requirements for medical device labels.
- ISO 15223-1:2021 new symbols for medical devices
- FDA Labelling Requirements: An Overview. This provides a deep overview of the labelling requirements for United States, as per FDA regulations.
- ISO 20417:2020 : New ISO Standard on Information to be Provided by Manufacturer
- FDA Requirements for UDI on Medical Devices. This part provides a guideline for UDI for US market, including structure of the UDI, database registration and management of multiple data carrier in the label.
New Symbols for ISO 15223-1:2021
Firstly, these are the general new symbols:
- Symbols if the device is a Medical Device (MD) or In-Vitro Diagnostic (IVD).

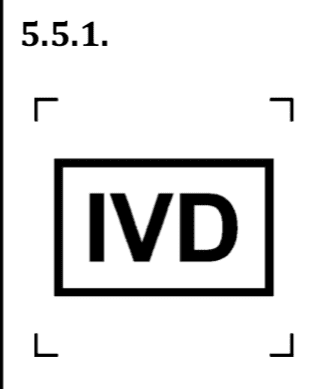
Moreover, for the IVD symbol, it is important to mention that the symbols is to identify in-vitro diagnostic devices and not medical devices to be used in vitro.
- Symbols to identify the repackaging of a device.
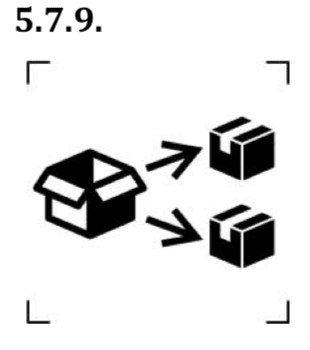
This symbol means that a modification of the original medical device packaging has been performed. The name and address of the responsible for repackaging activity shall be mentioned adjacent to the symbol. This symbols is used only when the repackaging is not performed by the manufacturer.
- UDI symbol.

The UDI symbols is optional. When multiple data carrier are present on the label, the symbol may be used.
- Name of the patient (for particular medical device).
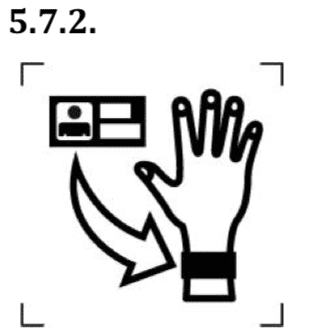
The symbol indicates the name of the patient
- Symbols to inform that some information for use are available on the web.
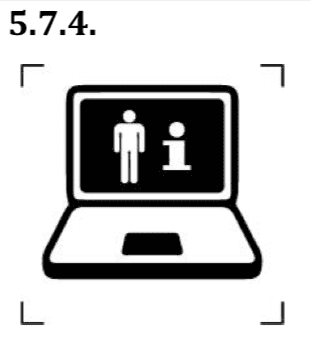
This symbol indicates that a patient can obtain additional information on the medical product. This symbol shall be accompanied by the web address adjacent to the symbol.
New Symbols related to Economic Operators
Furthermore, there are two symbols to identify economic operators under EU MDR and IVDR
- Importer
- Distributor
For instance, the EU MDR 2017/745 specifies in details the role of these two economic operators within European Union.
QualityMedDev has recently published a detailed checklist listing all the requirements related to labelling, information for use and packaging according to EU MDR 2017/745. This checklist helps to have all the requirements under control and provide an efficient method for their implementation, to ensure to respond to all the requirements mentioned in the European Medical Device Regulations.
New Symbols for sterile medical devices
Moreover, there are new indications to identify the type of sterile barriers for sterile medical devices:
- Singe Sterile Barrier System
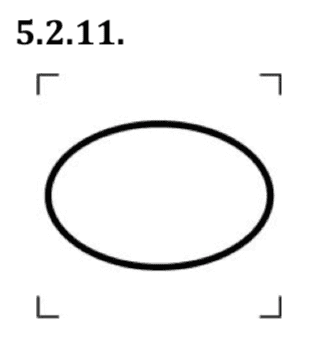
The symbol indicates a single sterile barrier system.
- Double Sterile Barrier System it is a very similar to the previous symbol, but it contains two lines and not one.
- Singe Sterile Barrier System with protective packaging inside
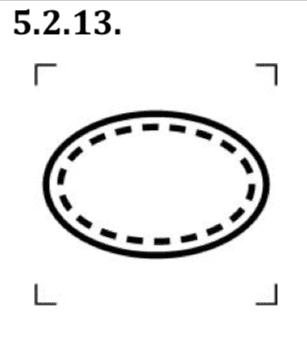
Specifically, this symbol indicates a single sterile barrier system with protective packaging inside. The protective packaging located inside the sterile barrier system prevents damage to the contents or to help with aseptic presentation. It does not provide a microbial barrier to maintain sterility.
- Singe Sterile Barrier System with protective packaging outside. This is a very similar symbol respect the previous one, but the dotted line is external to the continuous line. The protective packaging outside the sterile barrier system prevents damage to the sterile barrier system and the contents. The protection is against physical hazards, particulate contamination or other environmental hazards, but it does not include a microbial barrier.
Combination devices
Furthermore, there are as well new symbols to warn the user about biohazard-related risks.
Specifically, there are new symbols for medical devices containing:
- human blood or plasma derivatives;
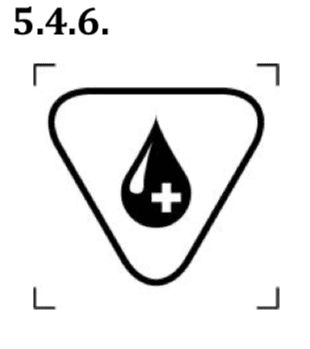
This symbol indicates a medical device that contains or incorporates human blood or plasma derivatives.
- a medicinal substances;
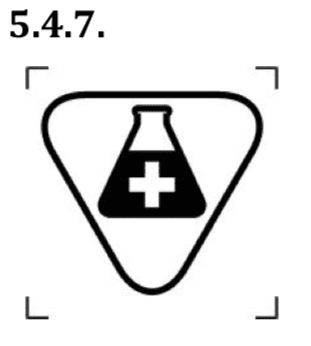
This symbol indicates a medical device that contains or incorporates a medicinal substance.
- biological material of animal origin;
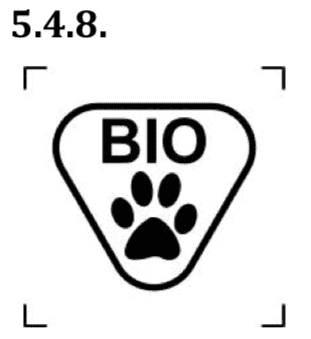
The symbol indicates a medical device that contains biological tissue, cells, or their derivatives, of animal origin.
- biological material of human origin.
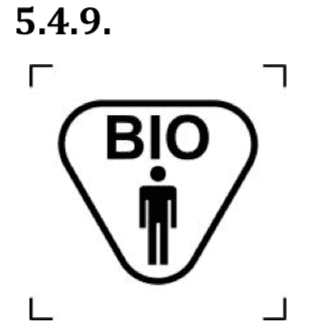
This symbol indicates a medical device that contains biological tissue, cells, or their derivatives, of human origin
This is just a high level overview of the new symbols for labelling of medical devices introduced with the update of ISO 15223-1.
Specifically, the publication of the revised standard will occur in 2021 along with ISO 20471 that will deal with information to be provided by medical device manufacturers.
To conclude, this is in the contest of waiting some future updates for ISO 13485 which could take big advantage from this new specific ISO standard. Furthermore, for an overview of ISO 13485 requirements, see this post on QualityMedDev. Do not forget to have a look to our WhitePaper on Biocompatibility Evaluation for Medical Devices.
QualityMedDev has also published an overview of the main labelling requirements for the FDA, including UDI and labelling for IDE devices and the requirements associated to the management of IFU in an electronic format (eIFU)
Subscribe to QualityMedDev Newsletter
QualityMedDev is an online platform focused on Quality & Regulatory topics for medical device business; Follow us on LinkedIn and Twitter to stay up to date with most important news on the Regulatory field.
QualityMedDev is one of the largest online platform supporting medical device business for regulatory compliance topics. We provide regulatory consulting services over a broad range of topics, from EU MDR & IVDR to ISO 13485, including risk management, biocompatibility, usability and software verification and validation and, in general, support in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy provides the possibility to follow online and self-paced training courses focused on regulatory compliance topics for medical device. These training courses, developed in collaboration with highly skilled professionals in the medical device sector, allows you to exponentially increase your competencies over a broad range of quality and regulatory topics for medical device business operations.
Do not hesitate to subscribe to our Newsletter!

