The role of post-market surveillance for medical device manufacturers became more and more important. In this post we give an overview to ISO 20416:2020, a new technical standard with the almost updated regulations for post-market surveillance.
The new ISO 20416 is complementary to other important medical device standards, e.g. ISO 14971 and ISO 13485 and the scheme below there is evidence of the interrelationships between the different standards:
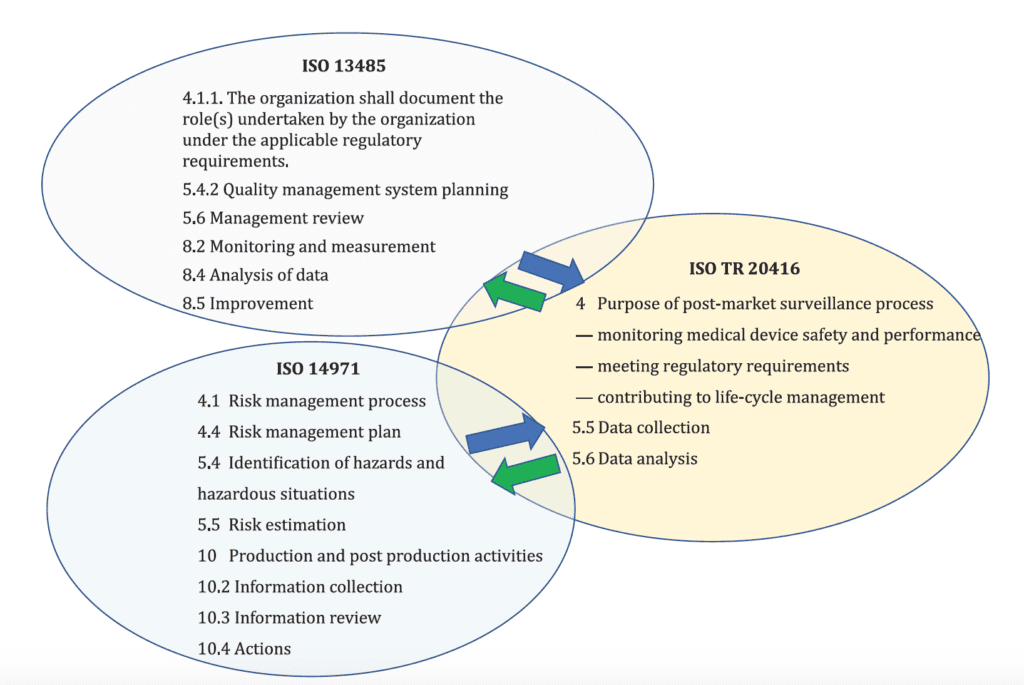
For this reason, this post focuses on specific aspects of post-market surveillance (PMS) that are outlined in the new ISO 20416. Specifically, the topics covered are:
- The purpose of post-market surveillance
- The objective of post-market surveillance
- Methodologies for data collection.
The purpose of Post-Market Surveillance
Firstly, both ISO 13485:2016 and ISO 14971:2019 require the implementation of processes for collection and analysis of data for production and post-production activities. Furthermore, post-market surveillance represents inputs for different processes, for example risk management or design and development.
In general, we can classify the purposes of post-market surveillance in three different categories:
- Monitoring Medical Device Safety and Efficacy: the collection of data related to production and post-production activities help manufacturer to have evidence of the safety and efficacy of the devices on the market. It also provide a framework for the implementation of corrective actions when safety and efficacy of the device is compromised.
- Meeting Regulatory Requirements: post-market surveillance activities can help manufacturers in meeting regulatory requirements by identifying safety -related issue very soon. An active PMS processionals can further support the requirements related to vigilance reporting by identifying adverse trends at the early stage.
- Contributing to life-cycle management: PMS can also to evaluate wether the medical device is not current state of the art and trigger design changes or changes in the intended use or user populations.
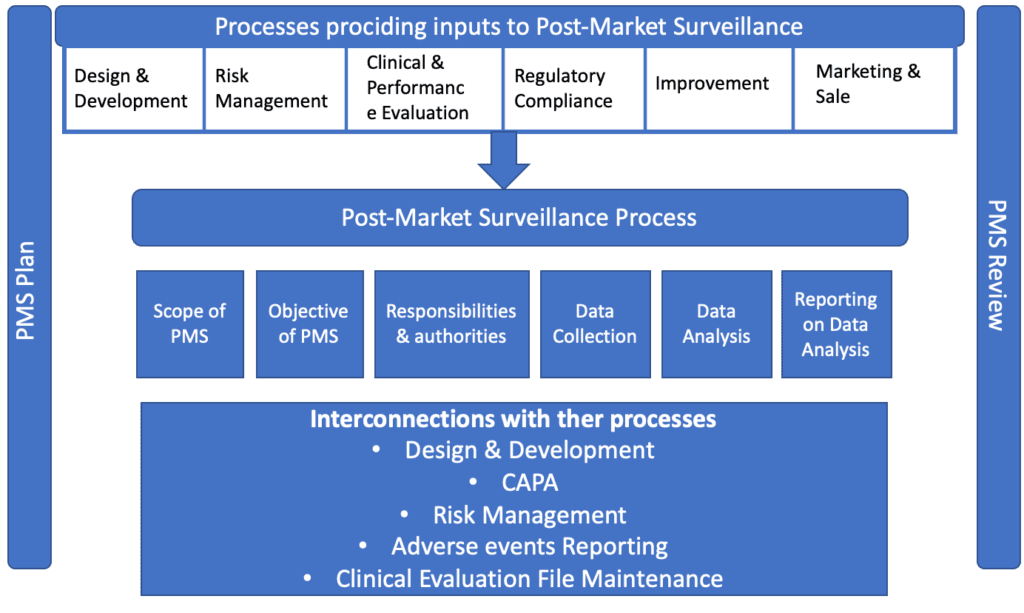
Objective of Post-Market Surveillance
Secondly, as stated in the new ISO 20416:2020, the main goal of post-market surveillance is to limit and reduce as much as possible the level of uncertainty about the safety and efficacy of the device on the market. Furthermore, the PMS plan defines the objective of PMS activities. Specifically, the PMS plan shall specify the type and adequacy of the information to be collected to ensure safety of the device on the market.
Moreover, the standard provides a series of questions that could help to better identify the objective of PMS activities. As a matter of example, I report here below some of the questions:
- are there any new hazards or hazardous situation identified for the medical devices or similar medical devices?
- any new misuse occurred with the devices?
- are there any unforeseen side effects for the medical devices?
- does the medical device need any improvements?
- can user or patient training reduce the likelihood of malfunction ?
- ect.
Methodologies of Data Collection
First of all, the organization shall define the source of the data used for PMS activities . The data collected should be precise and reliable enough to cover the objective of the post-market surveillance activities defined in the PMS plan. Moreover, the organization needs to consider data quality and integrity before using the specific data for PMS activities.
Furthermore, after having identified the source, the organization defines the method for data collection There are two categories of data collections for the manufacturers:
Specifically, examples of proactive collections of data are:
- written or electronic surveys
- interview of users
- literature search
- post-market clinical follow-up studies
- recall information and other information released by regulatory agencies.
Examples of reactive collections of data can b the review of
- Complaints
- non-solicited observations by healthcare professionals
- service reports
- regulatory compliance notifications.
Furthermore, for the correct selection of the appropriate method, there are other topics to consider. Specifically:
- the analysis method of the collected data
- sample size of the collected data
- the goal of the method
The Data Collection Protocol
Finally, after having defined the collection method, the organization prepares a Data Collection Protocol, that defines all the steps required to ensure consistency of the collected data.
Moreover, the Data Collection Protocol shall define how the data collection is managed, how data are record and related responsibilities, the monitoring of the collected data, how quality and integrity of collected data is ensured and the responsibilities for quality and integrity.
In conclusion, post market surveillance is currently playing an important role for medical device manufacturers and it is an essential tool for demonstrating quality and efficacy of the device on the market. This new standard ISO 20416 have been recently published along with a lot of new and updated medical device standards:
- Risk management standard ISO 14971:2019
- Labelling : ISO 15223:2021 and QualityMedDev E-book
- Information to be supplied by manufacturers ISO 20417:2021
- Clinical Evaluation ISO 14155:2020
QualityMedDev E-Book on Post-Market Surveillance
QualityMedDev recently published e-book of 36 pages that provides a practical guideline to post-market surveillance activities according to EU MDR 2017/745. This document represents a comprehensive overview of the medical devices PMS requirements for the European market. After all the changes introduced through the MDR, there has been the necessity of one unique document that explains and discusses the medical device post-market surveillance requirements.
Specifically, the topics covered by the e-book are:
- ISO 20416:2020 and the New Requirements for Post-Market Surveillance Activities for Medical Devices
- Deep Dive in the Post-Market Surveillance (PMS) Processes according to EU MDR 2017/74
- The Post-Market Clinical Follow-up
- Requirements Periodic Safety Update Report according to EU MDR
- Template for Periodic Safety Updated Report according to European Medical Device Regulation
- Vigilance Reporting Requirements according to EU MDR 2017/745
This Guideline on Post-Market Surveillance (PMS) Activities provides an overview of new processes such as PMCF (Post-Market Clinical Follow-up) and PSUR (Periodic Safety Update Report) and it includes as well an example of PSUR template. Moreover, an overview of the vigilance reporting system according to EU MDR 2017/745 is discussed.
