Over the course of time, the ISO 2859 standard has established itself as an indispensable tool, not only for medical device manufacturers, but also for a wide range of manufacturing companies that rely on effective sampling methods grounded in statistical principles to enhance their operational processes.
In our previous discussions, we have extensively explored the application of statistical methodologies within the context of a comprehensive Quality Management System. It becomes increasingly evident that the implementation of the Acceptable Quality Level (AQL) sampling methodology is crucial, as it serves as one of the fundamental statistical tools required for ensuring product quality and conformity within the organizational framework.
To be more specific, the ISO 2859-1 standard holds a prominent position as the internationally recognized reference for AQL sampling inspections. By following the guidelines outlined in this standard, manufacturers can establish a systematic approach to conducting sampling inspections based on attribute-based criteria.
Now, let’s delve deeper into the most significant sections of the ISO 2859-1 standard, which will enable us to gain a comprehensive understanding of its key requirements and their implications for quality control and assurance within various industries.
Acceptance Activities and QMS Requirements according to ISO 2859
If we consider the ISO 13485:2016 there are specific requirements associated to the verification of purchase products, specifically in the section 7.4.3. Basically the requirements are related to the necessity to have a system for the verification that purchased products are aligned with the specifications defined with the supplier. As usual, a risk based approach shall be applied and thus the extent of the verification activities shall be based on the results of the supplier evaluation and proportionate to the risk associated with the purchased products.
If we consider the MDSAP, specific requirements for acceptance activities should also be taken in consideration. For example, Anvisa Regulation requires evidence that sampling plans are based on valid statistical rationale. The organization should also periodically review the sampling methods to ensure they are consistent and suitable for their intended use. The frequency of the review of the sampling plans should be based on the occurrence of nonconforming products, audit results and complaints records.
Very similar requirements are mentioned in the US 21 CFR 820 Code of Federal regulation, thus the FDA regulation for Quality Management System. In fact, in the section in the section 21 CFR 820.250(b), it is reported:
Verify that the manufacturer establishes and maintains procedures to ensure that sampling methods are adequate for their intended use and ensure that when changes occur, the sampling plans are reviewed.
Before starting with the discussion on the requirements mentioned in the ISO 2859-1, it is important to report some definitions, focusing on the words which are not frequently used and thus the meaning might not be clear to everybody in the framework of Acceptance Quality Limits. These definitions are directly taken from the ISO 2859.
- Inspection by attributes
inspection whereby either the item is classified simply as conforming or nonconforming with respect to a specified requirement or set of specified requirements, or the number of nonconformities in the item is counted. - Sampling plan
combination of sample size(s) to be used and associated lot acceptability criteria. - Sampling system
collection of sampling plans, or of sampling schemes, each with its own rules for changing plans, together with sampling procedures including criteria by which appropriate plans or schemes may be chosen. - Acceptance quality limit (AQL)quality level that is the worst tolerable process average when a continuing series of lots is submitted for acceptance sampling.
In the subsequent sections, as mentioned above, we will discuss the requirements associated to ISO 2859-1 with particular attention to the following topics:
- Acceptance Quality Limit
- Lot Acceptance Vs Not Acceptance
- Type of Inspections
- Sampling Plans
The Acceptance Quality Limit (AQL) according to ISO 2859
The Acceptance Quality Limit (AQL), in conjunction with the sample size code letter, plays a pivotal role in determining the sampling plans utilized during quality inspections. Essentially, these parameters govern the number of items to be examined and establish the maximum permissible number of non-conforming items that can be present for the entire lot to be accepted.
To put it simply, the AQL serves as a critical benchmark that guides the selection of an appropriate sampling scheme tied to a specific AQL value. This scheme ensures that the lot under consideration will be accepted if the observed quality level, represented by the percentage of non-conformities per 100 items, does not exceed the designated AQL value.
In practical terms, the AQL value signifies the predetermined quality threshold that delineates acceptable levels of non-conformities within a given lot. By adhering to the specified AQL value, manufacturers can make informed decisions about the acceptability of a batch based on the results obtained from the sampling inspection process. It provides a standardized framework for evaluating the overall quality of the lot while simultaneously taking into account the inherent variability associated with sampling.
By utilizing appropriate sampling plans based on the AQL, organizations can establish a robust quality assurance framework that strikes a balance between cost-effective sampling efforts and reliable assessment of product quality. The AQL serves as a crucial tool for decision-making, allowing manufacturers to determine whether a lot meets the predetermined quality standards, thereby ensuring consistent product quality and customer satisfaction.
Lot Acceptability according to ISO 2859
The determination of lot acceptability relies heavily on the chosen sampling plan, as it serves as the foundation for decision-making. If a lot is deemed unacceptable based on the sampling results, it is categorically rejected, rendering it unsuitable for use, particularly in the context of verification activities during incoming inspections.
Once a lot is rejected, the next step involves deciding how to handle its disposition. Several options exist, including scrapping the entire lot, subjecting it to rework to rectify any non-conformities, or resubmitting it for further inspection. In some cases, the decision to accept a lot may be reconsidered after conducting a thorough risk assessment of the non-conforming elements.
Similarly, when a lot is accepted, it is crucial to address any non-conforming items that may have been identified during the inspection process. These items can be either scrapped, reworked to bring them into compliance, or resubmitted for subsequent inspection. It is important to note that even if an entire lot is accepted, there remains the prerogative to reject individual non-conforming items found within the accepted lot.
In situations where a lot is rejected due to a significant number of non-conformities, it is imperative to communicate this outcome to all relevant parties involved. As per the guidance provided in ISO 2859-1, it is recommended that rejected lots be subjected to re-inspection until every item has been thoroughly re-examined, ensuring that all non-conforming items have been effectively eliminated or appropriately reworked. In some instances, it may be decided to employ a more stringent inspection approach during the re-inspection of the previously rejected lot.
The type of Inspections
The ISO 2859-1 defines three level of inspections, named:
- Tighten inspection
- Normal Inspection
- Reduced Inspection
Usually, at the beginning normal inspection shall be used. Then, it is possible to change the type of inspection performed based on the results of the inspection previously performed, according to the scheme reported below:
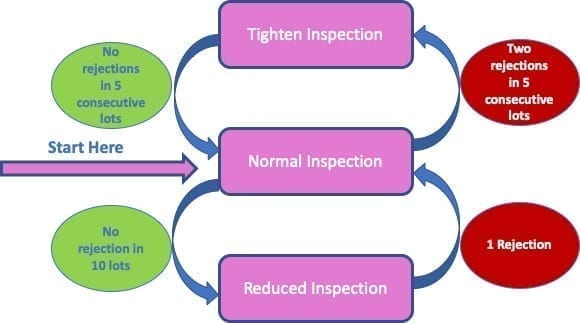
The switching rules between different levels of inspection are reported in the scheme above. When normal inspection is performed, tightened inspection shall be performed when two out of five consecutive lots have been rejected or considered non-acceptable. Of course, it is possible to switch the level of inspection even before reach the level of five consecutive lots.
At the same time, when tightened inspection is performed, it is possible to return to normal inspection level when there are no rejections in 5 consecutive lots.
In some circumstances, it is also possible to decrease the level of inspection from normal to reduced. This is typically feasible when reduced inspection is desirable (for example to avoid cost issue on destructive samples) and the production is at steady rate. To switch, usually there might not have been rejections for 10 consecutive lots.
When we are at the reduced level, it is necessary to return a normal inspection level in case of:
- A lot is not accepted
- Production becomes irregular
How AQL Tables are Used reported within ISO 2859?
There are different tables that need to be taken in considerations to determine the sample size to be inspected and the maximum number of non-conforming items it is possible to have to do not reject the lot under inspection.
Firstly, it is necessary to look at the Sample Size Code Letters, as reported in the table mentioned below.
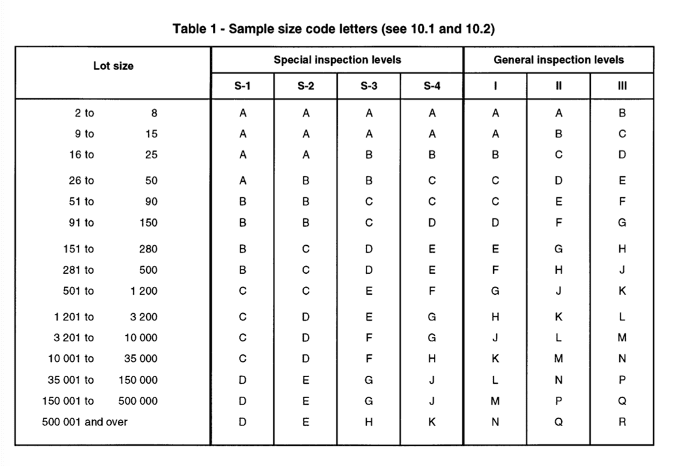
As it can be seen, it is necessary to select the appropriate inspection level, which basically, designates the level of inspection. Under standard circumstances, the general inspection shall be considered. Under this category, three level of inspections are considered. As it is mentioned within ISO 2859-1, unless otherwise specified, level II shall be used. Level I and III could be used when less or greater discrimination is needed.
Moreover, for additional special levels S1- S4 are defined in the aforementioned table and they can be used with relatively small sample size.
When the sample size code letter is found out based on sample size and inspection levels, it shall be used on other tables to find the related sampling plan. The table to be used depends from the level of inspection (normal, tightened or reduced).
For example, let’s imagine in the framework of incoming inspection control we have a lot of 1500 items. First, in the sample size code letters table we need to find the related code letter. If we consider we are using Type II General Inspection Level, the code letter is K.
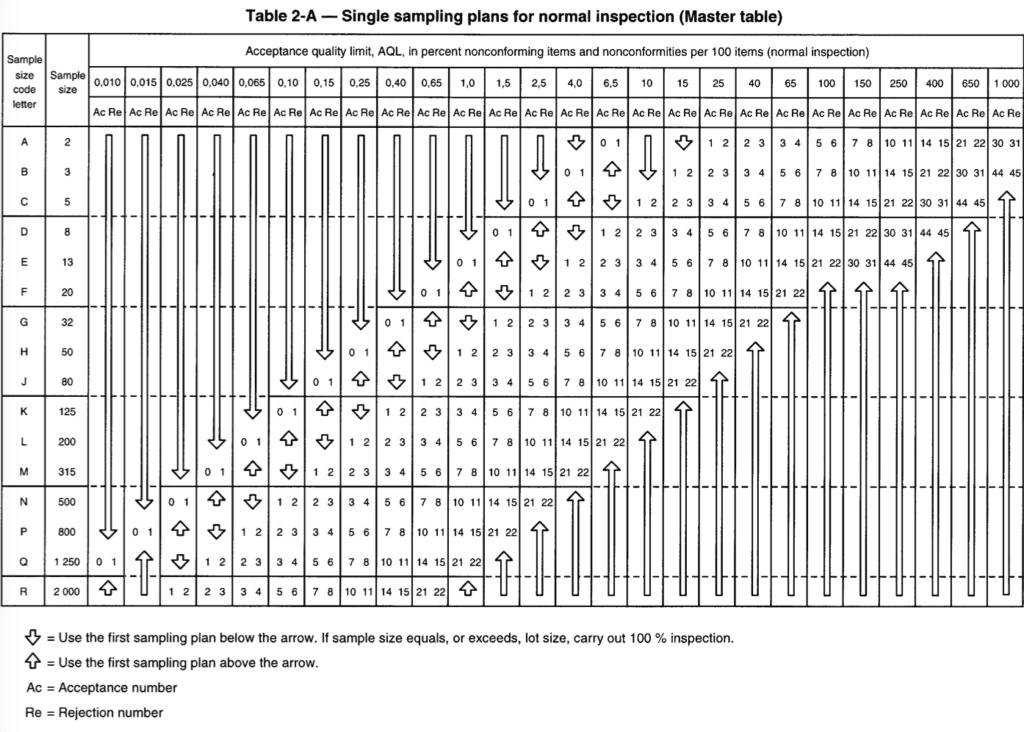
Then, if we consider we are using a normal inspection level, we should go to the related table (reported above) and we can find that the sample size code letter K corresponds to a sample size of 125. This number corresponds to the number of items to be checked in the framework of our incoming inspection activity.
Then we need to decide the AQL linked to the specific activity. As a reminder, the AQL corresponds to the number of non-conformities for 100 items. Let’s assume that our AQL for the incoming inspection of our lot sample is 2.5. If we check the table above, this AQL corresponds to a limit of acceptance of 7. It means we cannot have more than 7 non-conformities to accept the lot; thus if we have 8 or more non-conformity within the 125 items to be checked, the lot will have to be rejected.
Conclusions
In conclusion, we have provided a comprehensive overview of the ISO 2859 standard and its associated Acceptance Quality Limit (AQL) sampling methodology. It is important to note that the value of these concepts extends beyond the realm of medical device manufacturers, finding applicability in various industries.
When it comes to verifying purchased products, specific requirements are outlined in standards such as ISO 13485:2016 or in regulations such as the FDA Quality System Regulation and RDC ANVISA 16/2013 (Brazilian Regulation). These requirements often emphasize the necessity of employing a statistically valid methodology to support verification activities related to purchased products. The AQL sampling methodology, as elucidated in ISO 2859, is a widely used statistical tool that fulfills this need.
By implementing the AQL sampling methodology, organizations can ensure that their verification activities adhere to recognized statistical principles. This methodology enables them to establish appropriate sampling plans, determine sample sizes, and establish acceptable quality levels for purchased products. The AQL value assigned to a sampling scheme serves as a benchmark, indicating the maximum permissible level of non-conformities per 100 items that can be tolerated while still accepting the lot.
Moreover, the AQL sampling methodology promotes consistency and objectivity in the verification process. It provides a structured approach for evaluating the quality of purchased products, helping organizations make informed decisions about lot acceptability and subsequent disposition. This statistical tool is designed to strike a balance between the need for effective quality control and the practical constraints of inspecting every individual item in a lot.
In essence, the ISO 2859 standard and its AQL sampling methodology offer a robust framework for conducting verification activities on purchased products. They contribute to the overall quality management system of an organization by ensuring statistically sound and reliable inspections. Regardless of the industry, the utilization of these concepts can enhance the integrity of verification processes, leading to improved product quality and customer satisfaction.
Subscribe to QualityMedDev Newsletter
QualityMedDev is an online platform focused on Quality & Regulatory topics for medical device business; Follow us on LinkedIn and Twitter to stay up to date with most important news on the Regulatory field.
QualityMedDev is one of the largest online platform supporting medical device business for regulatory compliance topics. We provide regulatory consulting services over a broad range of topics, from EU MDR & IVDR to ISO 13485, including risk management, biocompatibility, usability and software verification and validation and, in general, support in preparation of technical documentation for MDR.
Our sister platform QualityMedDev Academy provides the possibility to follow online and self-paced training courses focused on regulatory compliance topics for medical device. These training courses, developed in collaboration with highly skilled professionals in the medical device sector, allows you to exponentially increase your competencies over a broad range of quality and regulatory topics for medical device business operations.
Do not hesitate to subscribe to our Newsletter!

