Gamma sterilization is extensively used in the life science sector and more specifically in the medical device sector, where gamma radiation plays a fundamental role as technique for product sterilization.
Gamma rays are able to delver higher energy respect x-ray and gamma rays are able to pass through substances like plastic and kill microorganisms like bacteria.
Gamma irradiation is known as a “cold process.” In fact, this type of sterilization is particularly suitable for rmed for rather sensitive or critical medical devices which could easily support a “cold sterilization process” such as gamma sterilization.
Needless to say that sterilization is a key process for medical device with direct impact on the safety of the devices; the sterilisation process has interconnection with other important processes such as clean room validation, cleaning validation and packaging validation. Particular attention to the validation of the gamma sterilization (and in general for any type of sterilization process) shall be given, in order to ensure no safety issue linked to the sterility of the device are observed from the devices on the market.
What is Gamma Sterilization?
The gamma irradiation used for sterilization is rather straightforward. It basically uses Cobalt 60 radiation to kill microorganisms. From technical standpoint, gamma radiation is generated by the decay of the radioisotope Cobalt 60, with the resultant high energy photons being an effective sterilant.
The absorbed dose for the gamma radiation is expressed in kiloGray (kGy). The absorption capacity of a specific device towards the gamma radiation is dependent from different parameters, such as the size of the packaging, the density of the product.
Cold Sterilization Process
Until some decades ago, the main sterilization processes for medical devices was the so-called heat sterilization through autoclave. Then, the introduction of the so-called cold process took over respect heat sterilization; nowadays there are different cold process used for sterilization activities, such as Ethylene Oxide, Formaldehyde and, indeed, gamma radiation.
The gamma sterilization process is a chemical/physical type of sterilization , that is able kill microorganisms like bacteria through the breakage of bacterial DNA. At the end of the sterilization, process, gamma radiation does not leave any residue.
Validation Approaches for Gamma Sterilization
In order to use radiation-based sterilization, it is necessary to determine the dose at which a product will be irradiated. This could be performed mainly by following two different standards:
- ANSI/AAMI/ISO 11137-2 Sterilization of Healthcare Products- Radiation Part 2: Establishing the sterilization dose, or
- ANSI/AAMI/ISO TIR13004. Sterilization of health care products – Radiation – Substantiation of a selected sterilization dose: Method VDmaxSD.
There are different steps for the determination of the sterilization dose to be used to ensure sterility of the specific device; these steps can be described by the scheme below:
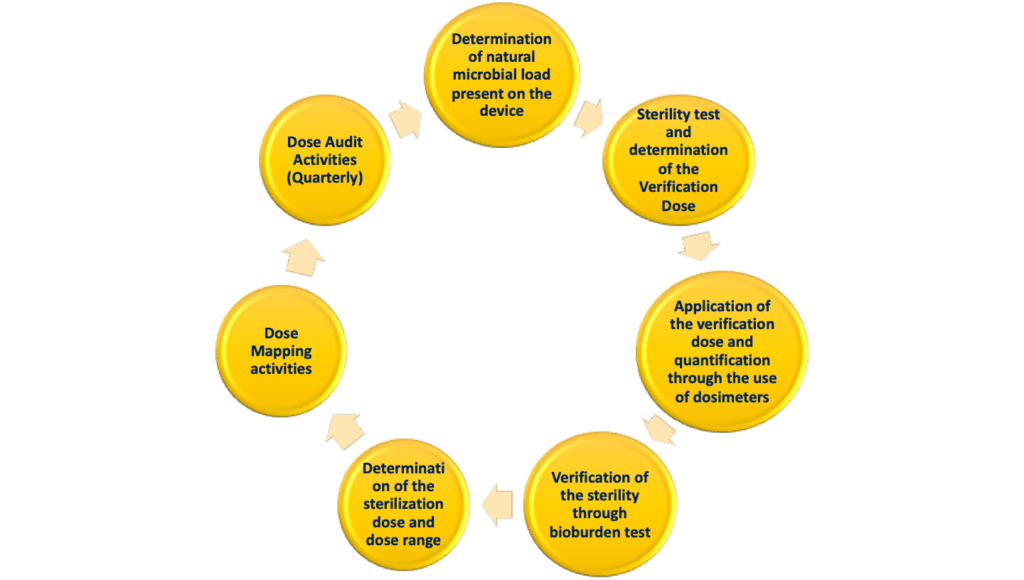
Here below a detailed explanation of each step.
- Determination of the natural microbial load present in the unsterilized packaging and device. In other words, it is necessary to determine, from a statistical standpoint, the natural amount of bacteria present on the device.
- The second step consists in the determination of the required radiation dose to apply to the product in order to achieve sterility with gamma sterilization. There is a specific table on the ISO 11137 that correlates the level of bioburden on the product and the dose to apply (in KiloGrays) to achieve a specified Sterility Assurance Level (SAL). The referenced SAL dose is termed the “Verification Dose”.
- Using specific dosimeters placed on the product, the verification dose is directly quantified. In this way it is possible to measure the amount of dose received by the product during the sterilization process. Since these tests are performed on the single product, the dose necessary are very limited; so this test are typically not performed in an industrial setting.
- Ater the application of the verification dose, the sterility of the device is evaluated in a microbial laboratory to ensure the product is indeed sterile and the verification dose has been efficient.
- Subsequently, the Sterilization Dose shall be determined in order to ensure the sterility of the device when it is tested in an industrial process. In this context, it is necessary to determine a minimum dose that it is necessary to use in order to achieve a determined level of sterility. Moreover, at this stage, it is also necessary to determine a dose range which can be used to sterilize the products. In this way, it is known that any dose within the specific range is able to impart sterility to the devices.
- When the sterilization process is performed based on a standard process, it is unnecessary to perform dose mapping activities. These are performed by selecting the best positioning of the product in a carrier and placing numerous dosimeters throughout the product load to establish the minimum and maximum areas of imparted dose.
- The sterilization dose shall be quarterly verified in order to establish its validity. This is performed through the so-called audit and a dose audit shall be determined and compared with the sterilization dose. An audit should be performed following any change that could significantly affect the level or nature of the bioburden.
Evaluation of the microbial load: Bioburden Test
When dealing with sterile medical device, it is essential, in order to keep the process under control, to perform evaluation of the microbial load on a device. This is done to ensure the parameters used in the sterilization process are adequate to ensure the sterility of the product.
The bioburden test is a well know microbiological test used to evaluate the number of bacteria living on a specific surface. By performing the bioburden test on a specific medical device before and after sterilization, it is possible to fully demonstrate the efficacy of the sterilization process.
Usually the bioburden requires a validation method step, where a certain amount of bacteria are placed on a device and then removed using the same method that would be used for the actual bioburden test. Based on the outcome of the method validation, a recovery factor is determined to account for the percentage of microorganisms that were not able to be removed from the device.
The bioburden test is extensively used in gamma sterilization validation for the determination of the microbial load of a device.
Conclusions
In conclusions, we went through the main concepts of gamma sterilization, including as well some high level consideration on the approaches to be used for validation of gamma sterilization process.
Sterilization is a key process for sterile medical device and it is essential to have a precise idea of the main requirements associated to this type of process as well as the requirements associated to the validation of this type of process.
Subscribe to 4EasyReg Newsletter
4EasyReg is an online platform dedicated to Quality & Regulatory matters within the medical device industry. Have a look to all the services that we provide: we are very transparent in the pricing associated to these consulting services.
Within our WebShop, a wide range of procedures, templates, checklists are available, all of them focused on regulatory topics for medical device compliance to applicable regulations. Within the webshop, a dedicated section related to cybersecurity and compliance to ISO 27001 for medical device organizations is also present.
As one of the leading online platforms in the medical device sector, 4EasyReg offers extensive support for regulatory compliance. Our services cover a wide range of topics, from EU MDR & IVDR to ISO 13485, encompassing risk management, biocompatibility, usability, software verification and validation, and assistance in preparing technical documentation for MDR compliance.
Do not hesitate to subscribe to our Newsletter!
